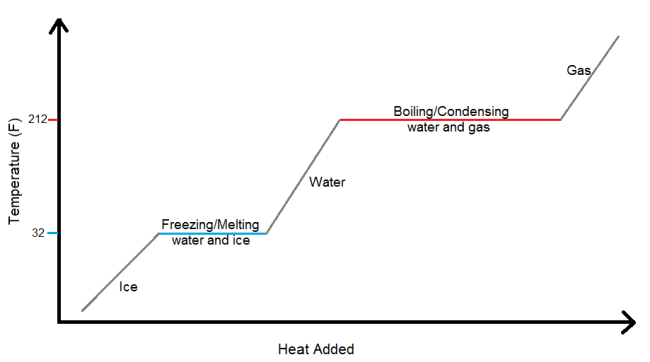
Summer’s coming up, which means it’s time to get out your coolers, fill them with ice and refreshments, and head out into the sunshine. But after a couple of hours, half the ice in your cooler will be melted. Does that mean that your drinks aren’t as cold as they would be if the cooler were full of only unmelted ice? No worries. As long as there is still any ice at all in your cooler, your drinks continue to chill at 32°F. At first, it seems more intuitive that melted ice means the temperature in the cooler is rising, but that isn’t entirely true. If the ice started out colder than the freezing point of water (32°F), then the ice will have warmed somewhat in order to reach 32°, but once it’s at 32° the temperature stays constant until every last ice cube has melted. There’s a diagram below to help illustrate this – basically, the temperature of the water cannot rise another degree until all of the ice is converted into water (the flat part of the graph at 32°). So even though the sunshine continues to add heat to the ice-water, that heat is used to convert the ice into water and not to raise the temperature. This is because adding energy (in this case by adding heat, which is a form of energy) can do two things: raise the temperature, or convert the water from one state to another; it cannot do both at the same time.
The same property holds true when water continues to heat up and is converted to a gas. Let’s say you start with water at room temperature (approximately 70°F) and want to boil it so you can cook some pasta. You turn on the stove, which proceeds to add heat to the water, thereby increasing its temperature one degree at a time until the water reaches 212°F. It’s still just water at this point, albeit increasingly hot water. Once at 212°, the water begins to boil. You may have never thought about it before, but once it’s boiling, the water is essentially constant at 212° because it takes a lot of heat to convert all of the water into gas even after the average temperature in the pot has reached the boiling point.
Thus far, the examples I’ve used have focused on water changing from ice to liquid water to a gas, but the graph shown above (it’s called a heating curve) applies to every substance, regardless of what state it is in at room temperature. Any solid substance, such as gold or plastic, can be heated until it melts into a liquid; cold enough temperatures can force things like nitrogen, which are a gas at room temperature, to condense from a gas into a liquid. And these other substances follow the same rules that water did – when they are at their freezing/melting or condensing/boiling point, the temperature of the substance stays constant until the entire group has changed from solid to liquid or liquid to gas. The shape of the heating curve remains the same in every case, but the freezing and boiling points and the amount of heat needed to reach them will change depending on the substance.
So next time someone worries that they need to add more ice when half the ice has melted, let them foolishly run off to find more while you coolly grab yourself another beverage, safe in the knowledge that it will still be cool and refreshing – and allow yourself to feel smug because you know why that is true.

You are amazing.
Pingback: Energy: In motion and at rest | Ease Into Science